
ALL HYPOCHLOROUS SOLUTIONS ARE NOT CREATED EQUAL

SETTING THE STANDARD
Born out of necessity, The Hypochlorous Company manufacturers the highest standard in HOCl. Our patented and innovative technologies allow us to offer the purest formulations for any industry or application. We believe Hypochlorous needs to be available universally to rid the world of toxic chemicals. With the killing power of Hypochlorous we set the standard for shelf life, kill time, certifications, and accessibility.

WHAT IS HYPOCHLOROUS?
Hypochlorous acid is a weak acid that is naturally produced by the human body as a part of the immune system. It is created by white blood cells and is used to kill bacteria and other pathogens that can cause infections. It is also used in disinfectants and cleaning products as a way to kill germs and bacteria on surfaces.
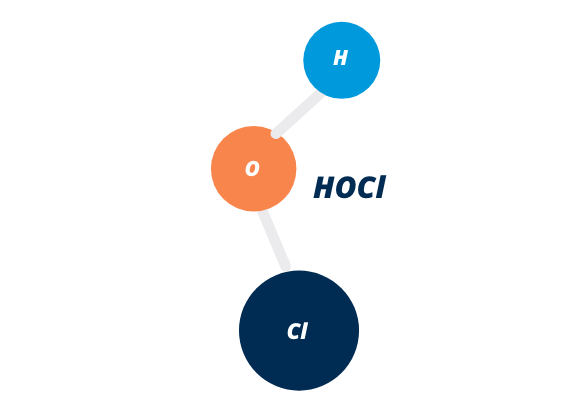
HOW DOES HYPOCHLOROUS WORK?
Hypochlorous acid works by disrupting the cell walls of bacteria and other microorganisms, which causes them to die. It does this through a process called oxidation, which involves the transfer of electrons from one molecule to another. When hypochlorous acid comes into contact with bacteria or other microorganisms, it donates electrons to them, which causes their cell walls to become damaged and eventually leads to their death. This makes it an effective disinfectant and can help to prevent the spread of illness.
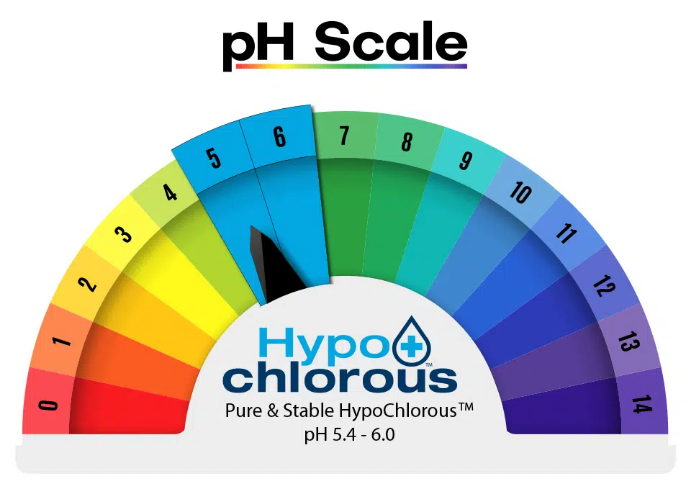
THE IMPORTANCE OF PH
Hypochlorous acid is a weak acid, less acidic than other organic substances such as orange juice or even a ripe banana.
The term “weak acid” means that when dissolved in water the molecule can partially dissociate into a positively charged Hydronium ion (H3O+) and a negatively charged Hypochlorite ion (OCl–). The degree of dissociation is a function of the pH of the solution. The Hypochlorous molecule is most effective and is the safest when it has the lowest amount of dissociation at a pH of between 5.4 and 6.0.
-
EFFECTIVENESS OF HYPOCHLOROUS SOLUTION
The hypochlorous solution rapidly loses effectiveness if the pH of the solution is too low or too high. If the pH is too low the hypochlorous molecule is transformed back to the chlorine molecule and can form chlorine gas. If the pH is too high, the hypochlorous molecule dissociates into the Hypochlorite molecule, which is ineffective as a cleaner and disinfectant and can begin to cause damage to surfaces and health.
-
PROVIDING SUPERLATIVE PH CONTROL
In summary, pH control is of critical importance. Hypochlorous™ Solutions and Systems have been formulated and designed to provide superlative pH control — the best in the industry. For our systems we even formulate different Hypochlorous Additives to consider the pH and alkalinity of the water source used to make the Hypochlorous™ Solution.
-
OUR HYPOCHLOROUS ADVANTAGE
In contrast, other systems, such as hypochlorous system made in China and distributed in North America, do not take pH into account. Because the pH of the source water used to make the product can vary significantly from one municipality to another, the quality of the resultant hypochlorous solution made with that source water can vary widely. So the hypochlorous made by such systems without pH control can, and likely is, outside of the optimal pH range and will not be very effective as a cleaner and disinfectant.

STRICT STANDARDS
Products are stored in opaque bottles to block out sunlight, and label directions call for the products to be stored in a dark closet at room temperature.
Assuring minor impurities do not accelerate the reversion.
The Hypochlorous Company uses a unique set of specialized, purified ingredients that are not available to the public, to assure that minor impurities do not accelerate the reversion of the Hypochlorous solution back to its dilute saltwater state. And finally, The Hypochlorous Company judiciously uses important stabilizers to assure the stability of the solution pH and to extent the shelf life of our products.
-
APPROPRIATELY DEFINED SHELF LIFE
One last word on stability and shelf life. Some competitors have defined shelf life in such a way that they can start with a solution concentration at 600 parts per million (ppm) and then let it degrade over a year or two to a solution concentration of 200 ppm, and then declare that they have a shelf life of two years, because the solution has not degraded below 200 ppm.
-
EPA TESTED UNDER EXTREME CONDITIONS
Comprehensive studies conducted by EPA laboratories under extreme temperature conditions have confirmed that the Hypochlorous™ Solutions produced by The Hypochlorous Company have a minimum shelf life of four months.
